Prospective Phase 2 investigator-initiated clinical trial results announced
[by Yu, Suin] Findings from a study evaluating the efficacy of the immunotherapy pembrolizumab in patients with locally advanced thymic epithelial tumors have now been published. Recognized as the first globally meaningful clinical investigation conducted specifically in thymic cancer patients, the study was selected for an oral presentation at the European Society for Medical Oncology (ESMO 2025) and was simultaneously published in the Journal of the International Association for the Study of Lung Cancer.
A research team at Samsung Medical Center, led by Professor Park Se-hoon of the Department of Hematology and Oncology, Professor Park Seong-yong of the Department of Lung and Esophageal Cancer Center, and Noh Jae-myung of the Department of Radiation Oncology, announced on November 18 that they have recently published the results of a Phase 2 clinical trial evaluating pembrolizumab + chemotherapy combination therapy for patients with locally advanced thymic epithelial tumors. The findings are officially published in the Journal of Thoracic Oncology (IF=20.8), the official journal of the International Association for the Study of Lung Cancer.
This study represents the world’s first clinical evidence demonstrating the efficacy and safety of preoperative combination therapy with the immunotherapy pembrolizumab in patients with thymic epithelial tumors, followed by a two-year course of postoperative maintenance therapy.
The thymus gland, a butterfly-shaped organ located beneath the sternum (breastbone), plays a protective role for the heart and lungs. It operates as a key component of the immune system until puberty, after which it undergoes involution and gradually becomes replaced by adipose tissue in adulthood. As with any organ of the human body, malignant transformation can occur, leading to the development of cancer within the thymus. Thymic epithelial tumors are extremely rare, with an annual incidence of less than one per 100,000 individuals. Nonetheless, recent trends indicate a rising occurrence. A report jointly presented by Samsung Medical Center and the National Cancer Center at the 2022 World Conference on Lung Cancer showed that the incidence of thymic epithelial tumors in Korea has been increasing at an average annual rate of 6.1%.
Thymic epithelial tumors typically are asymptomatic and are frequently identified incidentally during routine health checkups, including chest X-rays or CT imaging. As with many malignancies, early detection allows for curative surgical intervention. In cases where surgery is not initially feasible, neoadjuvant chemotherapy may be administered to reduce tumor size and increase the likelihood of surgery. However, the limited efficacy of standard chemotherapy regimens, characterized by response rates of only 20-30%, has posed a significant clinical challenge.
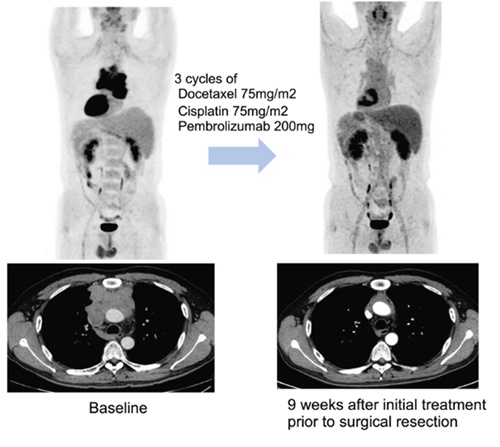
Accordingly, between March 2020 and January of this year, the research team recruited 40 patients with thymic epithelial tumors in a single-arm, investigator-initiated prospective clinical trial. The study reported that the majority of participants (33 patients, 82.5%) presented with stage IV disease, deemed unresectable at diagnosis based on the Masaoka-Koga staging system. Most participants (72.5%) have thymic carcinoma, a subtype associated with a particularly poor prognosis.
The research team administered pembrolizumab in combination with standard chemotherapy over three treatment cycles, each separated by a three-week interval, after which they re-evaluated candidates for surgical feasibility. Following surgery, pembrolizumab maintenance therapy was continued for up to 32 weeks, with some patients receiving additional chemoradiation.
Over a median follow-up period of 27.5 months, the research team observed a substantial tumor reduction in 57.5% of participants (23 patients) after the preoperative regimen. In addition, disease control, defined as the prevention of further tumor progression, was achieved in 82.5% of patients. Owing to these favorable treatment responses, including sufficient tumor reduction, 70% of the cohort (28 patients) ultimately became eligible for surgical intervention.
Moreover, 32.5% of participants (13 patients) demonstrated a major pathological response (MPR), achieving a 10% or less residual viable tumor on pathological assessment. Although this outcome did not meet the initial target of a 50% MPR rate, the figure rose to 46.4% among those who proceeded to surgery, underscoring the potential role of pembrolizumab in the treatment of thymic epithelial tumors.
"This combination therapy yielded a more favorable therapeutic response in thymic carcinoma, a subtype characterized by poor prognosis, than in thymic tumors, which generally exhibit relatively better prognosis among thymic epithelial tumors," said Professor Park Seong-yong. "Should surgical intervention become feasible for patients who were previously difficult to treat, even more favorable prognosis outcomes may be anticipated," he added.
Indeed, the one-year disease-free survival (DFS) rate among patients who proceeded to surgery reached a notably high 87.9%. The median DFS was 49.3 months, demonstrating that disease recurrence or progression was delayed for nearly four years.
Notably, because the median overall survival (OS) has not yet been reached within the study period, the prospects for long-term survival appear increasingly favorable. "Although this investigation was conducted as a single-arm study and therefore requires additional safety validation, it is nonetheless meaningful in that it provides an opportunity for complete cure in patients who are ineligible for surgery, particularly those with thymic carcinoma. We intend to conduct additional research in thymic carcinoma to further demonstrate the clinical efficacy of the treatment," stated Professor Park Se-hoon.

