[Interview] Kyu Baek, CEO and Co-Founder of Qureator
[by Kang, In Hyo] Entering clinical trials in new drug development signifies the point at which a candidate is first administered to humans. For this reason, pharmaceutical companies conduct rigorous preclinical evaluations, including extensive animal toxicity and safety testing, to ensure that a drug is sufficiently safe before progressing to human trials.
However, confirming safety in animal studies does not guarantee equivalent efficacy in humans. Due to fundamental differences in biological environments, it is common for therapeutic effects observed during preclinical testing to fail to translate into clinical trials outcomes.
Therefore, expectations across the industry are rising for systems that can evaluate drug responses in a ‘human-relevant’ environment before entering clinical trials, as such approaches are anticipated to improve clinical success rates. Against this backdrop, Qureator, headquartered in San Diego, California, has drawn significant attention. Qureator is the first company in the world to secure U.S. Food and Drug Administration (FDA) investigational new drug (IND) approval for a therapy based on organoid-derived, AI-driven ‘organ-on-a-chip’ treatment data, establishing itself as a pioneering artificial intelligence (AI) ‘organ-on-a-chip’ platform developer.
On October 6, SillaJen, a Korea-based biopharmaceutical company, received FDA approval for a revised IND application for combination therapy using its anticancer drug candidate, BAL0891, together with Tislelizumab, an anti-PD-1 immune checkpoint inhibitor developed by the global pharmaceutical company BeOne Medicines. The Korean Ministry of Food and Drug Safety (MFDS) has also approved the IND on September 9, based on the same supporting data. With this approval, SillaJen is now authorized to initiate a clinical trial aimed at evaluating the safety and determining the optimal dosage of the BAL0891 + Tislelizumab combination therapy.
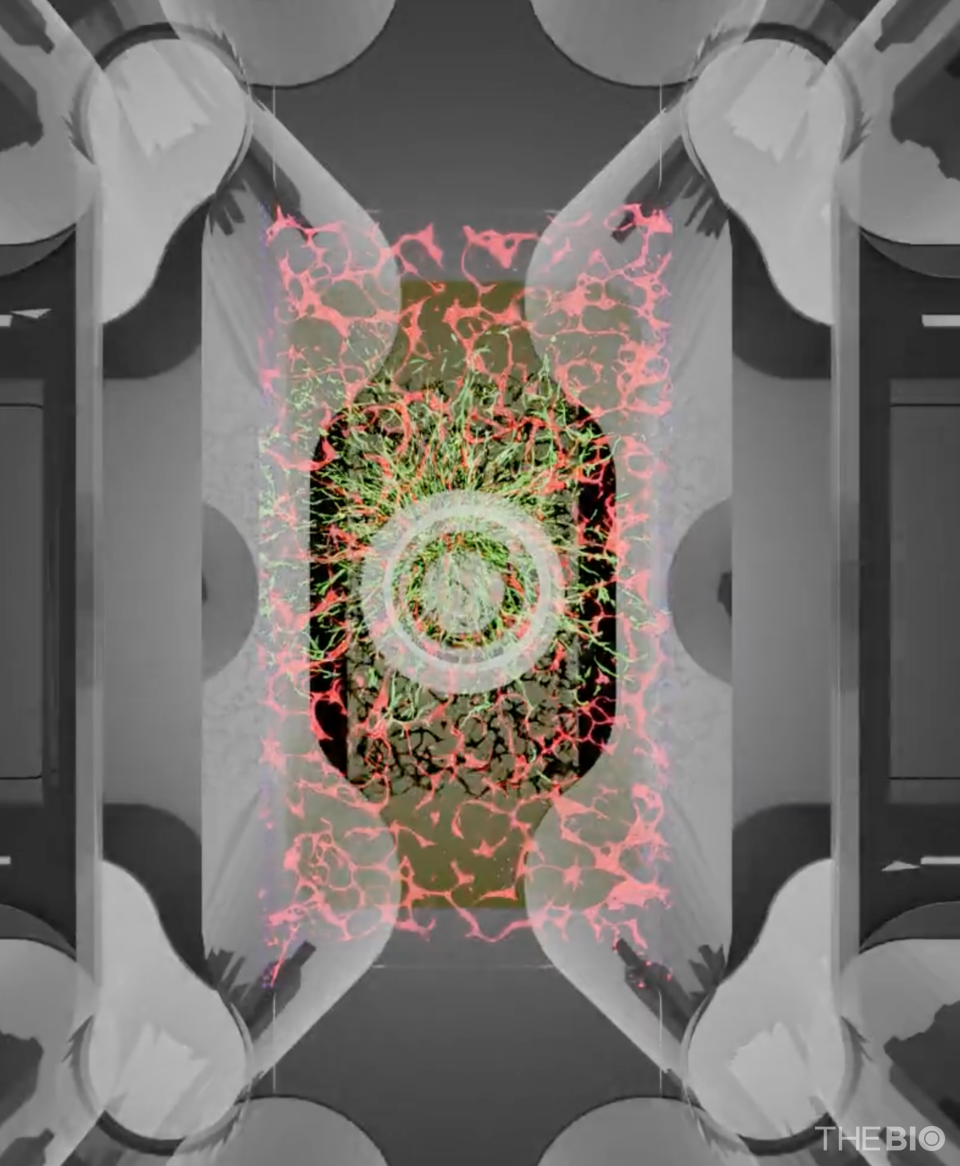
The pivotal factor behind this clinical approval was Qureator's ‘vTIME (vascularized Tumor Immune MicroEnvironment)’ technology. This platform, built on a 3D tumor organoid model that realistically replicates human vascular architecture and immune-cell responses, served as crucial evidence in validating the efficacy of the combination therapy during the preclinical stage.
Qureator CEO Kyu Baek, during a recent visit to Korea, stated in an interview with <THE BIO>, "We are the first in the world to demonstrate the efficacy of this combination therapy without relying on animal models, using only data generated from our own 'human-relevant' disease model, and to secure FDA approval on that basis." He emphasized, "This milestone shows that regulatory authorities and innovative companies can work together to shift preclinical evaluation from animal-based testing to human-based trials."
"For decades, the clinical failure rate in domestic novel drug development has remained extremely high, around 80-90%. In oncology alone, the annual economic loss attributable to clinical failures is estimated at KRW 60-70 trillion (approximately USD 40-47 billion)," Baek further noted. "Qureator aims to present a new paradigm for drug development in this difficult landscape. Through our innovative platform, we are committed to developing novel drugs with drastically reducing the enormous costs and timelines involved, and significantly lowering clinical failure rates."
In the interview with <THE BIO>, Baek elaborated on the company's founding story, overarching vision, and strategic direction for the future.

◇Founded on the ‘Vertex Success Story DNA’… Centered on a ‘human-relevant’ approach
Qureator positions itself as a ‘human-relevant’ AI-driven drug discovery company. By generating data in ‘human-like’ biological environments, such as organoids and organ-on-a-chip systems, the company has developed a platform capable of predicting drug responses without the need for animal testing. Qureator’s strategy center on training its AI models with this human-relevant data to enhance the efficiency of drug development and significantly improve the probability of clinical success.
Founded in 2019 in San Diego, USA, Qureator is now in its sixth year of operation. The founding team is composed entirely of former researchers from the global biotech company, Vertex Pharmaceuticals (hereafter referred to as Vertex), an important detail, given Vertex’s deep historical ties to the organoid field. Vertex was the first pharmaceutical company to collaborate with Hans Clevers, the Dutch Utrecht University professor known as the ‘Father of Organoids.’ Clever pioneered the development of intestical organoids, and Vertex became the earliest company to apply these ‘human in vitro models,’ which closely mimic in vivo biological environments, to evaluate drug efficacy during the preclinical stage. This collaboration marked the first practical application of Clevers' ‘intestinal organoid’ technology into drug discovery.
"The Qureator founding team consists of core members who shared this experience at Vertex. This background is deeply embedded in our DNA and enabled us, from the very beginning, to design a platform that seamlessly integrates organoids, tissue engineering, bioprocessing, and AI. This foundational integration is a major differentiator that sets us apart from other competitors in the field," Baek remarked.
Baek is a seasoned expert with more than 25 years of experience leading biomedical research, diagnostics, and medical device innovation at companies such as Vertex and Hospira. Qureator's technological and business strategy is overseen by Dr. Yoo Sang-hee, an industry veteran with extensive experience in oncology. Dr. Yoo brings over 20 years of cancer research expertise, having led anticancer drug discovery and preclinical development programs at organizations including Vertex and Tachyon. At Qureator, she heads the biological research organization and is responsible for driving platform-based anticancer drug development.
Dr. Branka Mitrovic, M.D., Chief Scientific Officer (CSO), is a seasoned research leader with more than 30 years of experience in developing novel therapeutics for genetic, autoimmune, and neurological diseases at companies such as Vertex and Bayer. She directs Qureator's drug discovery strategy and overall scientific direction. Dr. Tsung-Li Liu, Ph.D., a technology specialist with over 12 years of experience in biotechnology, advanced microscopy, and biomedical engineering at Vertex and Harvard Medical School, served as Qureator’s Chief Technology Officer (CTO). He is responsible for developing the company’s core technologies, including organ-on-a-chip systems and other human-relevant model platforms.
In addition to Baek, Qureator has another co-founder: Professor Noo-li Jeon of the Department of Mechanical Engineering at Seoul National University. Jeon founded the Korean biotech company Curiochips in 2016. As a research-based spin-off from Seoul National University, Curiochips developed an in vitro platform capable of mimicking three-dimensional vascular structures to create human organ-on-a-chip systems. The company received investment from Seoul Techno Holdings, with seed-staged participation from investors such as SparkLabs and Mirae Asset Capital, laying the foundation for its growth. Originally launched as a hardware-oriented platform company, Curiochips shifted strategic direction with the arrival of a new executive team, adopting an ‘integrated three-pillar strategy’ that combines in vitro models, data generation, and AI. Following this transition, the company completed a corporate restructuring, with existing shareholders maintaining their equity in Curiochips, which subsequently became wholly owned subsidiary under a newly created overseas parent company, a structure commonly referred to as flip, in which a domestic entity is placed under the ownership of a foreign holding company.
"By incorporating human-relevant disease models and AI-driven new drug discovery into our early hardware-oriented capabilities, we have transformed into a 'human-relevant, AI-based new drug discovery and development company,’” Baek said.

◇"Easing of animal testing regulations poised to reshape nonclinical strategies of novel drug developers, prioritizing 'human-similar data'"
In April, the U.S. Food and Drug Administration (FDA) issued new guidelines that exempt monoclonal antibodies (mAbs) from previously mandatory animal testing requirements during regulatory review. This policy shift reduces the reliance on animal studies in mAB development and is expected to broaden to other areas of drug development, leading to changes in nonclinical research strategies in the biopharmaceutical industry.
Furthermore, the U.S. National Institutes of Health (NIH) recently unveiled a ‘new policy direction’ signaling its intention to actively expand the use of ‘New Approach Methodologies (NAMs)’ as alternatives to traditional animal testing. The NIH expects that NAM-based evaluation systems will become established as ‘alternative methods’ capable of complementing, or, in some cases, fully replacing conventional animal studies.
According to Baek, the NAMs, the alternative testing framework proposed by the U.S. NIH, is built on three core pillars. The first is cell- and tissue-based ‘in vitro’ models. The second is ‘in silico’ modeling, which relies on computational simulations and data-driven analyses. The third pillar comprises ‘new-omics’ technologies, including genomics, transcriptomics, and proteomics, which generate high-dimensional biological data. Collectively, these three components are regarded as essential technologies capable of replacing or complementing traditional animal models.
However, despite significant progress, in silico modeling have produced only limited practical outcomes. Although AI-based drug design is rapidly advancing, as of last year only a very small number of AI-designed compounds had successfully progressed through all stages of clinical development and demonstrated meaningful results.
"In recent years, AI-based drug discovery has produced a substantial number of candidate compounds. Some experts attribute this to improved preclinical prediction accuracy, particularly in areas like toxicity assessment," Baek explained. "Although AI is steadily advancing in safety-related (toxicity) prediction, many observers note that AI still faces clear limitations in 'efficacy prediction,' an area where high expectations were initially placed."
"Although NAMs technologies are rapidly expanding, each approach still faces inherent structural limitations. Major challenges include the inconsistent reproducibility of in vitro models, the discrepancy in predictive accuracy of in silico models, and the complexity and interpretive difficulty of omics-based data," he further added.
Baek explained that in vitro models, including organoids and organ-on-a-chip systems, use patient-derived cells to recreate human tissue environments and therefore provide a far more advanced evaluation platform than conventional single-cell assays. While these models offer a clear advantage by more faithfully reflecting human physiological, Baek emphasized that standardization and reproducible data generation are essential prerequisites for their successful application in the industry.
A second major limitation of in vitro models is the challenge of scale-up. Drug discovery requires extensive, repeated testing, yet current in vitro-based models often struggle to consistently generate reliable data when scaled up. "Although organoid and organ-on-a-chip technologies provide a high level of sophistication, they still face clear constraints when it comes to the standardization and consistency demanded in large-scale drug discovery and development settings," Baek noted.
Recently, there has been a rising demand for multimodal AI approaches that integrate in silico models with omics datasets. By incorporating large-scale genomic, transcriptomic, and proteomic information into training, these models aim to enhance the accuracy of predictive performance. However, Baek emphasizes that the fundamental limitations of current AI systems remain unresolved. This is largely because many AI-based drug design platforms still depend heavily on legacy 2D cell line-based datasets, that is, experimental data generated from conventional flat-plate cultured cells, as their primary learning source.
"For this reason, AI-generated candidate compounds are not sufficiently grounded in human-relevant data that truly reflects the physiological environment of the human body, which leads to recurring shortcomings in efficacy prediction," Baek expressed. "Although multimodal AI is certainly the direction the fields must move toward, predictive accuracy will remain fundamentally limited as long as the input data itself is disconnected from the authentic human biological environments."
"With the recent rapid advancement of spatial genomics, technology that analyzes thin tissue sections to map single-cell distribution and molecular characteristics, there is active research aimed at understanding biological organization at the single-cell level. However, because the therapeutic efficacy of novel drugs is ultimately determined by 'system-level interactions,' that is, complex biological responses involving multiple cell types, tissues, and interconnected signaling networks, information obtained solely at the single-cell-level has inherent and clear limitations," Baek further added.
Some experts argue that as long as analyses are confined to the cellular level, they cannot fully capture the intricate tissue- and system-level interactions that occur in the actual human body. For this reason, Baek emphasized that enhancing the predictive power of AI models requires building datasets redived from ‘human-relevant tissue models’ that more faithfully approximate actual biological environments. "Cell-based models are valuable, but drug action is ultimately determined at the system level, where interactions among tissues and organs converge," he emphasized. "To improve the accuracy of AI-based prediction, the incorporation of more human-relevant tissue models is essential."
Qureator's core strength lies in its capability to generate human-relevant disease models that can be scaled up while consistently producing high-quality, reproducible data. "Our platform enables the generation of uniform, reliable datasets, allowing us to overcome both the reproducibility challenges inherent to existing in vitro models and the limitations observed in current in silico and omics-based approaches," Baek explained.
"To overcome the scale-up issue, long regarded as the most significant limitation of in vitro models, we have developed proprietary hardware and an automation system that can repeatedly generate highly reproducible data. With our internally developed human-relevant disease model, we are able to produce stable, high-quality experimental data on a continuous basis," he further remarked.
"These datasets will be continuously integrated into our AI-based drug design platform, which is currently under development. In addition, we are incorporating multi-omics data derived from patient samples to further strengthen the model's predictive accuracy," he emphasized.
In other words, Baek noted that Qureator's self-developed human-relevant disease model will serve as the central foundation of future AI-based drug design platforms. Without a platform capable of realistically simulating the human physiological environment, it would be impossible to generate the level of clinical relevant data required to accurately predict how drug candidates will behave in the human body.
From this perspective, Baek explained that SillaJen's IND submission, mentioned earlier, illustrates the successful acquisition of a reproducible and reliable in vitro model. He noted that “achieving consistent data-generation capability at the early nonclinical stage will translate directly into competitiveness in AI-based drug development.”
◇"Human-like data must reflect not only physical similarities but also functional responses"
Baek stressed that human-relevant data is indispensable for improving the accuracy of AI-based drug design. While clinical data obtained directly from actual patients represents the ideal form of human-relevant information, it is inherently limited as a single patient provides only a narrow set of drug-response patterns, making it impossible to capture the broad spectrum of responses required for model training.
Therefore, industry attention is increasingly shifting toward the use of human-relevant models that simulate the human physiological environment, enabling the rapid and cost-efficient accumulation of drug-response data under conditions closely resembling those of actual patients. Baek explained that the continuous acquisition of data generated in human-like environments is key for training AI systems to learn and predict more realistic biological responses.
“While we stress the importance of the ‘simulation’ of human physiological conditions, the more fundamental criterion is the extent to which a simulation model can generate outcomes that truly mirror those of real humans,” he noted. “A model’s structural resemblance alone is insufficient; its functional responses to drug administration must also align closely with human biological behavior.”
"Therefore, functional validation is essential to determine how accurately the human-relevant model reproduces true human physiological responses," he further added. "The model's resemblance must be demonstrated through empirical results, and a systematic verification process is required to confirm that its drug-response patterns accurately reflect human biological responses."
To address the limitations of conventional organoids, Qureator has developed a platform that advances modeling to the tissue level. The company has engineered vascularized organoids in which a functional blood vessel-like network enables the natural infiltration of immune cells into the tissue. In addition, by embedding stromal cells around the organoids, Qureator has created a system that closely reproduces the tumor microenvironment (TME) found in humans. A representative example is the ‘vTIME’ platform, which was used in preclinical studies supporting the mentioned SillaJen's IND submission.
Indeed, Qureator has already demonstrated meaningful outcomes in its functional validation efforts. "When we compared responses to first-line chemotherapy using cells derived from multiple patients, our model's predictions were aligned with the actual patient outcomes at a rate of approximately 90%. These findings provide critical evidence supporting the scientific validity of our platform," Baek emphasized.
"Our second validation involved FDA-approved anticancer drugs. As is well known, certain drugs are ineffective against specific cancer types. We applied these drugs to our disease models and were able to replicate the clinically known lack of efficacy with 100% accuracy," he continued. "The ability to currently identify ineffective drugs is powerful evidence of the model's predictive reliability."
Qureator emphasized that its competitive edge extends beyond disease-modeling technology to include its manufacturing and production systems. Baek explained that the company maintains exceptionally high reproducibility and consistency throughout the production process, which in turn strengthens the competitiveness of the data generated from its platform. "AI platforms trained on such high-quality data naturally gain a competitive advantage. The company's core strength lies in a virtuous cycle of 'manufacturing capabilities → data quality → AI performance,'" he said.

◇“Expanding disease model-based collaborations across companies… Enhancing data competitiveness through a ‘flywheel’ effect”
Qureator's vTIME model is a 3D tumor organoid platform that accurately reproduces actual human vascular architecture and the surrounding immune environment. By enabling a more realistic simulation of drug penetration, distribution, and immune interactions than conventional organoids, vTIME is regarded as a technology that enhances clinical predictive power during the preclinical stage.
"SillaJen's IND approval is particularly meaningful because it passed FDA approval based on functional efficacy data generated exclusively from a human-relevant disease model, without relying on animal studies to validate the anticancer combination therapy," Baek stated. "Qureator's human-relevant model also played a role in determining the dosing sequence, dosage, and treatment regimen during the development of SillaJen's combination therapy."
In other words, essential design components, such as the sequencing of drugs A and B, the day interval between administrations, and the appropriate dosage for combination therapy, were evaluated using Qureator’s disease-model data and subsequently incorporated in the IND submission.
Qureator emphasized that its platform becomes increasingly competitive as collaborations with diverse partners expand, based on disease models, creating a ‘flywheel effect.’ As more experiments and joint projects are conducted, the platform accumulates a larger and more diverse dataset. Importantly, both successful and failed data are incorporated into model training, enabling the AI system to continuously refine its predictive accuracy.
"This framework facilitates the discovery of novel targets, which then are used to design and validate new drug candidates. It can also support patient-stratification strategies in clinical trials," Baek remarked. "A system in which the entire platform grows stronger as data accumulates is a unique advantage, one that is rarely seen among AI-based drug development companies."
This year, only six years after its founding, Qureator has achieved a global first, obtaining FDA approved for a combination clinical trial based on organoid-derived research. The company is now preparing for a Series B funding round, following its previous total KRW 28 billion (approximately USD 19.1 million) of Series A funding. Of particular note, in October 2024, Qureator secured an addition KRW 8 billion in series A-3 investment from STIC VENTURES and Solidus Investment. This funding paved the way for the company to advance preparations for the clinical approval process of SillaJen's combination therapy, ultimately establishing the foundation for Qureator’s first FDA IND approval.
Qureator highlighted its human-relevant platform-based combination therapy data and its strategy for expanding into biologics as core messages at annual meeting of the Society for Immunotherapy of Cancer (SITC), held this month in National Harbor, Maryland.
The presentation showcased data from the combination therapy pairing SillaJen's investigational candidate with a PD-1 inhibitor. Qureator underscored that the efficacy of this regimen was demonstrated entirely through its proprietary human-relevant disease model, without the use of traditional animal models, highlighting the platform's direct contribution to clinical study design.
The company also plans to unveil results from its dual-antibody development program. Notably, its ‘VEGF x PD-1’ bispecific antibody is designed to bind VEGF near the tumor, promoting the influx of T cells into the tumor microenvironment, a mechanism that has recently attracted considerable attention from global biopharmaceutical developers.
Qureator is currently preparing to launch a new project with a global pharmaceutical partner. The company plans to allocate the funds secured through its upcoming Series B round toward three objectives: expand its platform to encompass additional cancer types; extending its human-relevant modeling capabilities to other disease areas; and enhance the value of partner companies' compound by conducting investigator-led clinical trials designed to validate the platform's clinical predictive performance.
"The human-relevant, AI-based drug discovery and development platform will ultimately transform the paradigm of novel drug development. This shift is undeniable, and the question of who implements it first will be decisive," Baek further commented.
"The human-relevant data accumulated by Qureator will become a core foundation for this change. Through this, we aim to help raise clinical success rates and accelerate the development of new drugs that ultimately actual patients," Baek said.

